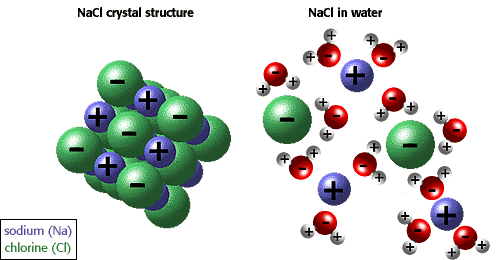
Why is the freezing point lowered when salt is added to water?
Salt is used to melt ice.Adding salt disrupts the equilibrium. The salt molecules dissolve in the water, but do not attach easily to the solid ice. There are fewer water molecules in the liquid because some of the water has been replaced by salt. This means that the number of water molecules able to be captured by the ice goes down, so the rate of freezing goes dow. The rate of melting of the ice is unchanged by the presence of the salt, so melting is occuring faster than freezing

No comments:
Post a Comment